Completed Trials
First-in-Man Safety Studies
- Because of the platform nature of the EDV technology, a number of safety trials with different therapeutic payloads were carried out in patients with solid tumors who had exhausted all treatment options
- Despite expected treatment- resistance, 50 out of 133 patients showed clinical benefit and the response rate was 37.5%
- Toxicity was low and patients maintained an excellent quality of life
- Overall survival was increased dramatically to >1year from commencement of EDV dosing
Not just a chemotherapeutic payload but delivering functional nucleic acids too
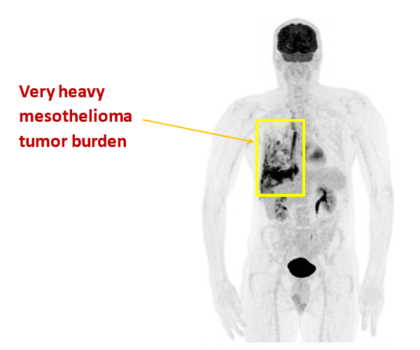
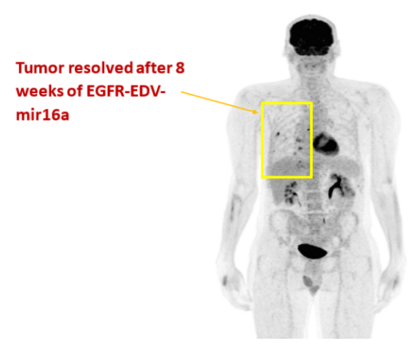
- A Phase I trial in patients with end-stage mesothelioma and who had failed standard therapies was conducted with EGFR-EDVs loaded with a microRNA 16a. The mir15/16 family is associated with unsuppressed cell growth when it is lost in malignant mesothelioma
- Despite this trial being a safety trial, those patients who completed at least one cycle of EGFR-EDV-mir16a (16 out of 22 patients) showed a clinical response and median survival was much longer than expected in this group of patients at 41 weeks post commencement of EDV treatment (Kao et al., American Journal of Respiratory and Critical Care Medicine 191(12): 1467-1469 (2015); Van Zandwijk et al, Lancet Oncology (2017)